Useto navigate. Pressescto quit
ELECTRO CHEMISTRY 3D model
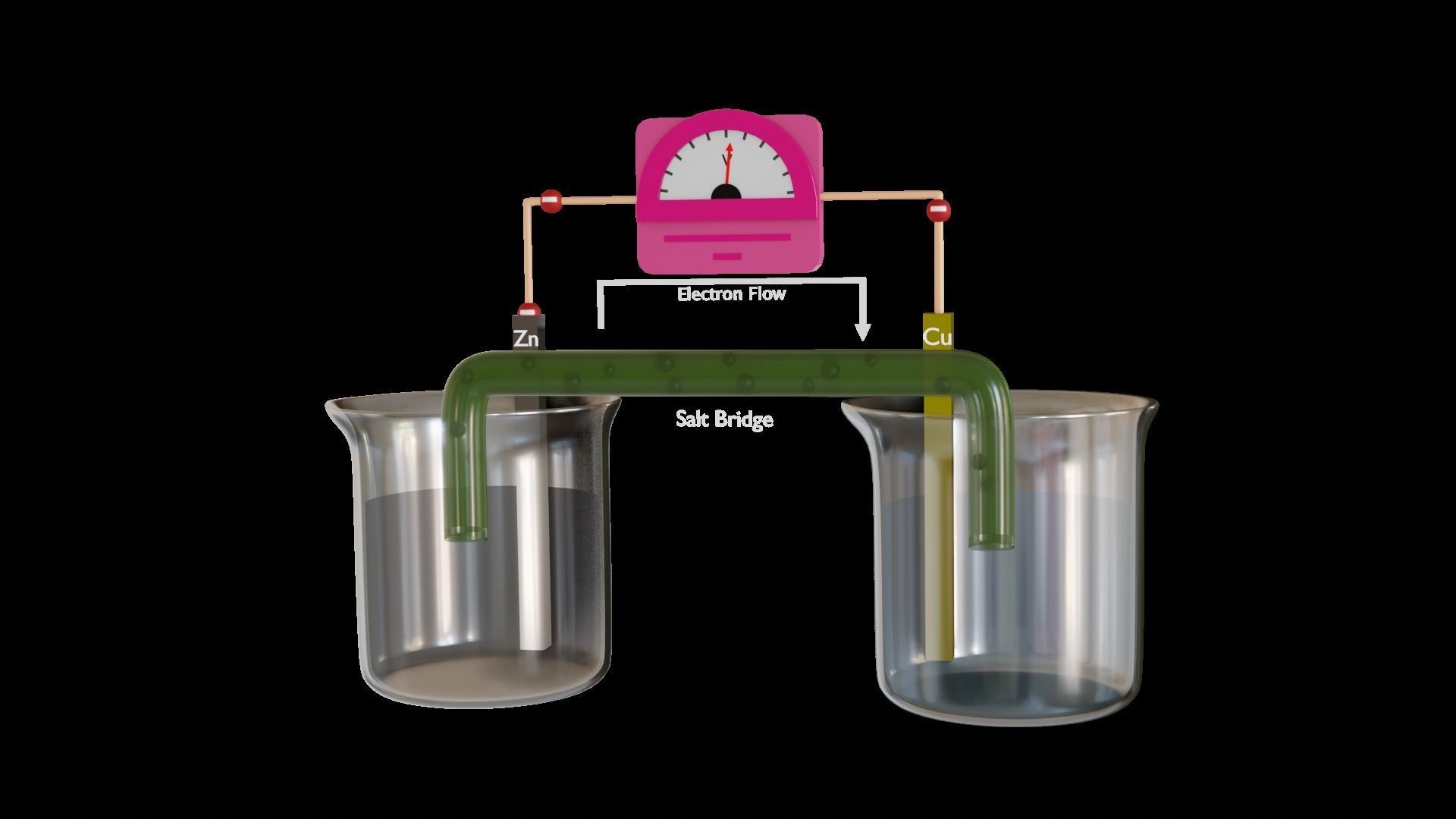
Description
An electrolytic cell is a cell that consists of positive and negative poles known as an anode and cathode. On the other hand, a galvanic cell is defined as an electrochemical cell that is used to convert the chemical energy of spontaneous redox reactions into electrical energy.